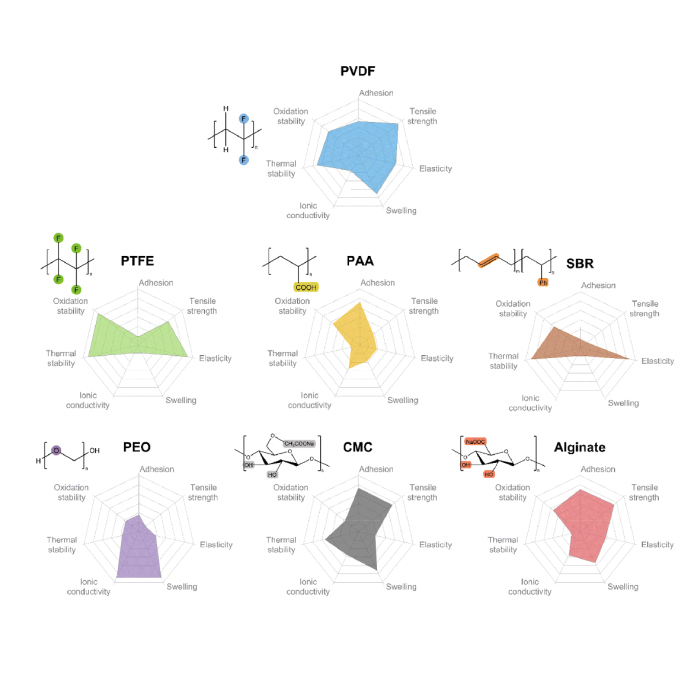
Comparison of Performance of Common Adhesives for Lithium Battery
The electrodes of lithium-ion batteries are mainly composed of active substances. binders, conductive agents, and current collectors. Although the content of binders and conductive agents is very low, they are crucial for the performance of lithium-ion batteries. The main function of the binder is to bond solid particles such as active substances and conductive agents into a whole, while also adhering the electrode coating to the current collector.
The functions of the adhesive include.
Provide mechanical stability. maintain the structure and volume changes of positive and negative electrode active materials during charging and discharging processes, prevent the active materials from falling off. And improve the cycling stability of the electrode plates.
Reduce the internal resistance of the battery, mix with conductivity, form a conductive agent network, and provide the required electron conduction inside the electrode:
Improve the wett ability of the electrolyte, adsorb the electrolyte, and promote the transfer of lithium ions at the electrode electrolyte interface
Performance requirements for lithium battery binders.
Adhesive performance: The adhesive must have characteristics such as good adhesive performance, high tensile strength, good flexibility, and low Young's modulus to provide sufficient adhesive strength, ensuring that during battery production and use (storage, cycling) there will be no active materials falling off the electrode during repeated expansion and contraction. and the bonding between electrode particles will not be damaged:
Chemical stability and electro-chemical stability: Under long-term high potential (positive electrode binder) or low potential (negative electrode binder) conditions, the positive electrode binder needs to not be oxidized under high pressure conditions, and the negative electrode binder needs to not be reduced under low pressure conditions; During storage and cycling (battery charging and discharging), the binder does not have any side reactions with active materials, Li, and other substances
Compatibility of electrolyte: insoluble in electrolytic solution or with low swelling coefficient, does not undergo chemical reactions with the electrolyte, and maintains stability in shape, structure, and properties in the electrolyte:
Processing performance: Good dispersion in the slurry medium, which is conducive to uniformly bonding the active substance onto the current collector and can provide good processing performance for the slurry, electrode, and battery:
Dynamic performance: has little impact on the negative effects of electron and ion conduction in the electrode.
Common adhesives used in lithium-ion battery electrodes include PVDF, PTFE, poly (acrylic acid) (PAA), styrene butadiene rubber (SBR), polyethylene oxide (PEO). sodium carboxymethyl cellulose (CMC), and alginate. The research team led by Yu Xiqian from the Institute of physics of the Chinese Academy of Sciences National Research Center for Condensed Matter Physics in Beijing compared the performance parameters of commonly used binders(PVDF,PTFE,PAA,SBR, PEOCMC,Aginate)
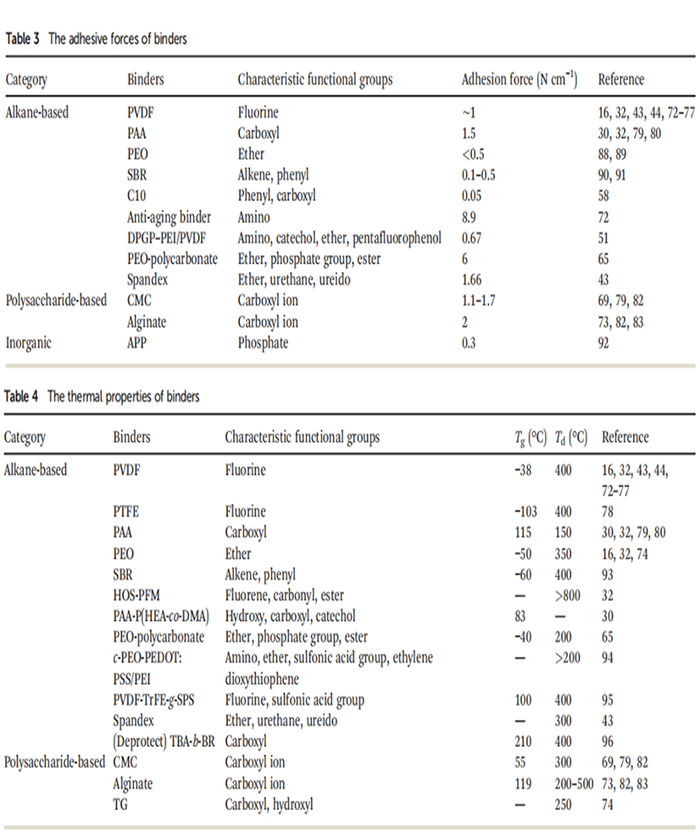
The basic properties of these adhesives, including tensile and compressive mechanical properties, adhesive strength, and thermal properties, are summarized in Tables 1, 2, 3, and 4, respectively.
By comparing the adhesion, tensile strength, elasticity, swelling ion1cconductivity, thermal stability, and oxidation stability of these seven common adhesives. their performance was evaluated and compared, as shown in Figure 1.PVDF exhibits better overall performance than other binders. PVDF is an important polymer, and its main large-scale manufacturing method is emulsion or suspension polymerization using vinylidene fluoride monomers, surfactants, and initiators. Compared to ethylene, the H atom in PVDF is partially replaced by the F atom.resulting in higher chemical, electrochemical and thermal stability of PVDF. The electrochemical window of PVDF is about 5 V. and the thermal decomposition temperature can reach up to 400 c. In addition, the molecular structure of PVDF is conducive to the formation of hydrogen bonds. which can ensure good mechanical. and adhesive strength through van der Waals forces and hydrogen bonding interactions with polymer chains or adhesive surfaces between molecules. Compared with fully fluorinated polytetrafluoroethylene (PTFE) PVDF exhibits higher tensile strength and adhesion. In addition, PVDF has good swelling behavior and crystallinity, so it can provide good ion conductivity after absorbing electrolytes. However, due to the low electron cloud density and polarizability of PVDF, the vander Waals force interaction between PVDF and other molecules is relatively weak. which is insufficient for the adhesion of ultra-high quality loaded electrodes (>20mgcm2) and also difficult to adapt to the large volume changes of the electrodes. The per fluorinated form of PTFE exhibits extremely unbalanced properties, and the PTFE adhesive contains CF2-CF2 units, exhibiting ideal chemical stability. Among these binders, mechanical and oxidative stability are the best, but adhesion and conductivity are poor. The cohesive force between adjacent chains in PTFE hexagonal crystals is low, making it easier to slide along the chain axis (the c-axis of the hexagonal system). Therefore. when a shear load is applied to the PTFE crystal, crystal slip occurs along the c-axis, and the shape of the PTFE crystal changes, forming a high aspect ratio nanofiber structure with a fiber diameter of only a few nanometers and a length of several tens of micrometers. By forming a three-dimensional network.
structure, the active material and carbon black aggregate together. Therefore, PTFE is often used in dry electrode processes.
In terms of mechanical properties, PAA, CMC, and alginate are not as good as PVDF, but they are water-soluble and rich in carboxyl or hydroxyl groups, which helps to form stronger adhesion. SBR has very high elasticity and is usually mixed with CMC to compensate for each other's shortcomings CMC is a multi-component weak acid that can dissociate to form carboxylate anion functional groups. Free carboxylate groups can interact with hydroxyl groups on the surface of materials such as silicon/carbon to form an ideal carbon gel phase network in the electrode. Moreover, CMC has low cost, good thermal stability, and is environmentally friendly. However, CMC water-based binders have strong rigidity and brittleness. After vacuum drying, cracks can be clearly seen on the surface of electrodes with CMC as the binder, which may even cause gaps between the electrode material coating and the current collector, resulting in electrode "material shedding". To solve this problem.butadiene styrene rubber (SBR) is often used as an elastic additive for CMC binder. The addition of SBR can effectively reduce the brittleness of the electrode. The Si anode using SBR-CMC composite binder shows smaller Young's modulus, greater maximum elongation, and stronger adhesion strength to the collector.PEO has excellent ion conductivity, but its oxidation resistance is poor under high voltage.